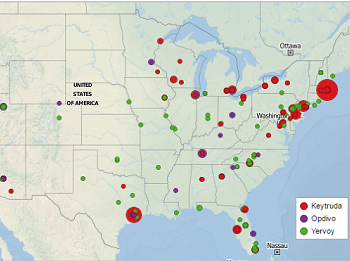
Location of top research centers for checkpoint inhibitors
Read moreImmunotherapies targeting CTLA-4 and PD-1 have recently garnered a lot of well-deserved press, as they continue to radically improve the outcomes of patients with cancer. Keytruda, Opdivo, and Yervoy are now all FDA approved across several tumor types and are being actively...
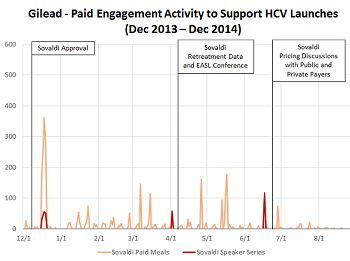
Gilead’s orchestrated HCV market development activity
Read moreGilead’s HCV franchise had a massive 2014, generating $12.4B in revenue for the full calendar year. Gilead was able to successfully introduce two new, curative therapies to the market despite a challenging pricing and reimbursement environment. While Gilead gets a lot...
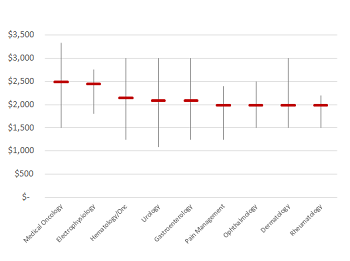
Benchmarking paid speaker fees by opinion leader specialty
Read moreSponsored speaker events remain an effective component of pharmaceutical marketing, particularly in building awareness around a new drug launch or clinical data release. While industry standards point toward an average fee of ~$1,750, we’ve found a considerable range amongst specialty areas. Using...
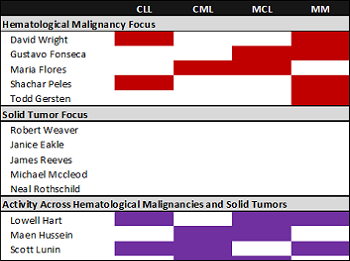
Characterizing clinical researcher alignment in Florida
Read moreCancer research is accelerating at a rapid pace and has begun to deliver clinically, with several breakthrough therapies recently winning FDA approval. In addition to advancing our understanding of cancer biology, strong relationships between drug developers and clinical researchers remains critical. In...
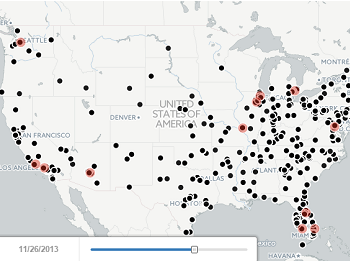
Tracking Roche’s next-gen anti-CD20 launch
Read moreRituxan (rituximab) is an incredibly successful drug for Roche, generating billions of dollars across multiple indications each year. Roche’s U.S. sales force interacts with 6,500+ physicians, with effort split primarily amongst oncologists (blue) and rheumatologists (red) as pictured below. However,...

